News
Cough Syrup Manufacturers Ordered to Conduct 100% Testing of High-Risk Raw Materials
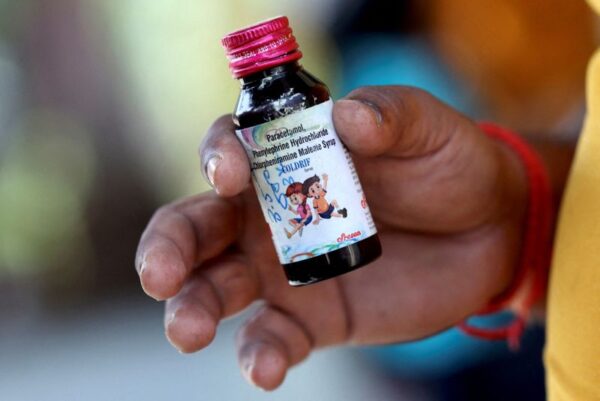
The state’s Health and Family Welfare Department has directed all manufacturers to ensure 100% testing of high-risk ingredients used in cough syrup production, as well as mandatory batch testing before commercial release, in order to strengthen patient safety measures.
The move follows the recent deaths of several children in Madhya Pradesh linked to the consumption of contaminated cough syrup. Officials said the new directive will help detect toxic impurities such as diethylene glycol (DEG) and ethylene glycol (EG) — both known to cause fatal poisoning — at an early stage of manufacturing.
The Drugs Control Administration (DCA) has identified a number of high-risk pharmaceutical excipients including DEG, EG, sorbitol, maltitol solution, and hydrogenated starch hydrolysate, where these impurities are often found. The department warned that failure to test these inputs at the start of production greatly increases the risk of contamination in formulations such as cough syrups.
In an order issued on October 9, State Drugs Controller Dr. Manish Kapoor instructed manufacturers to collect samples from 100% of excipient containers and test every batch of drug formulations like cough syrups before they are released to the market.
Authorities noted that many smaller manufacturers lack in-house quality testing laboratories and depend on private labs or even supplier-provided test reports without conducting independent verification — a practice that compromises consumer safety. In some cases, to save time and costs, manufacturers have skipped testing raw materials or finished batches before market release, increasing the risk of toxic products reaching patients.
To ensure the safety of pharmaceutical formulations, the DCA has set permissible impurity limits for these ingredients. Dr. Kapoor stated,
“The percentage of diethylene glycol (DEG) and ethylene glycol (EG) in excipients and final formulations must remain below 0.1%, as defined in their monographs. Manufacturers have been instructed to adhere strictly to these standards.”
Manufacturers have also been ordered to source excipients only from original producers or authorized distributors, eliminating the risk of adulteration through multiple layers of middlemen.
However, regulators observed that some companies buy cheaper industrial-grade excipients instead of pharmaceutical-grade ones to cut costs — a practice that can result in toxic formulations. Skipping mandatory quality checks further compounds the danger.
A pharmaceutical expert explained,
“Pharma-grade propylene glycol costs about ₹140 per kg, compared to ₹95 per kg for the industrial grade. By using the industrial version, a manufacturer can save millions of rupees per batch, but at the expense of public safety.”
